Rutgers' Massa makes supercomputer model to reveal details of declining lung function
SuperComputational approach could lead to better understanding of emphysema and other human lung diseases
Scientists have developed a new virtual model of mouse lung function that illuminates the relative importance of different factors that contribute to lung changes accompanying chronic inflammation. Christopher Massa and his colleagues at Rutgers University, New Jersey, present the work in PLOS Computational Biology.
Chronic inflammation, which is often found in people with chronic obstructive pulmonary disease (COPD), and aging both alter lung structure and function. Respiratory impedance--a measure of air flow and pressure in the throat--can be used to model and assess general lung function. However, this approach cannot distinguish the relative impact of specific changes, such as destruction of air sacs and altered airway size.
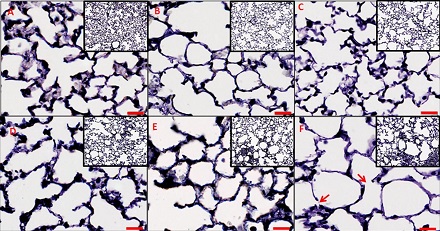
In pursuit of a more detailed approach, Massa and colleagues examined lung tissue and function in healthy mice, as well as in mice that had been genetically engineered to mimic conditions seen in people with emphysema, a type of COPD. Then, they used this experimental data to build a new model that simulates changes in mouse lung function, as indicated by respiratory impedance.
The researchers used the model to compare the relative influence on lung function of several factors associated with chronic inflammatory disease. These included lung tissue destruction, changes in density of elastic fiber structures that aid in stretching and contraction of lung walls during breathing, and changes in lung recruitment, in which elevated pressure keeps open lung regions that might otherwise collapse.
Modeling results suggested that changes in lung recruitment and elastic fiber density were mainly responsible for the observed decrease in lung function associated with chronic inflammatory disease in the mice. This contracts with previous proposals that tissue damage and loss of structure have the greatest impact.
The new model provides a fresh approach for examining how pathological changes alter lung function. "The most exciting aspect of this research is that by conducting these analyses we can determine what has happened to the lung tissue simply by measuring air flow and pressure at the throat," says study co-author Andrew Gow.
Further work will be needed to refine the new model and determine how the results might apply to humans. Nonetheless, it opens up new pathways for research. "The next steps for this research are to directly test how well the model can predict the effects of a therapeutic intervention, such as budesonide, upon lung function," Gow says.
