Unlock the full potential of CO2 capture with minimal molecules, the revolutionary solution to creating a greener future
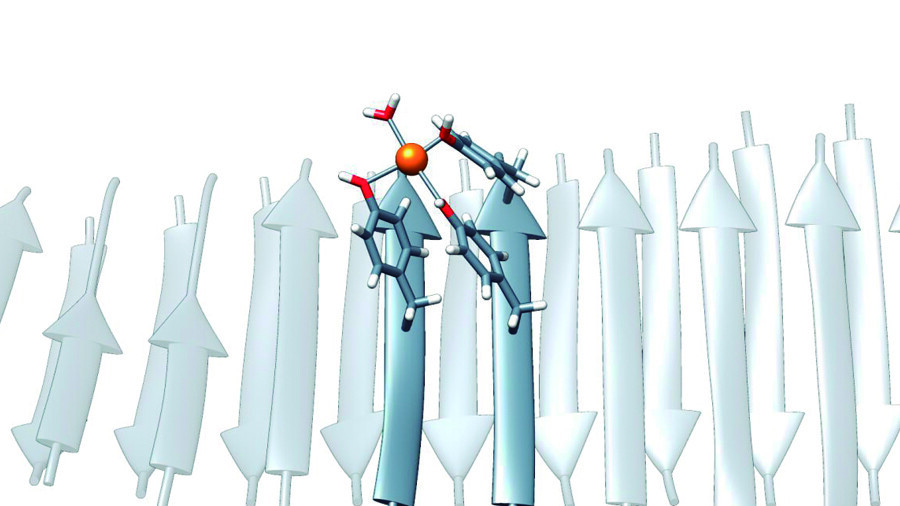
Researchers at the Universitat Autonoma de Barcelona (UAB) have developed enzymes capable of capturing carbon dioxide (CO2) emitted in industrial processes and other environmental remediation processes. These enzymes are based on artificial molecular structures formed by peptides of only seven amino acids. The new molecules can also act as metalloenzymes, which opens up new possibilities in biotechnology research. Furthermore, the study provides a new contribution to the origin of catalytic activity at the beginning of life.
The study was coordinated by Salvador Ventura, and Susanna Navarro was the first author. Both are researchers at the Institute of Biotechnology and Biomedicine and the UAB Department of Biochemistry and Molecular Biology. They collaborated with researchers from the UAB Department of Chemistry and the Research Centre bioGUNE.
In the study, the researchers used a combination of experiments and simulations, including spectrophotometry, fluorescence, electron microscopy, electron diffraction, and supercomputational modeling.
In 2018, researchers at UAB successfully created short molecules that can self-assemble. These molecules were inspired by the natural ability of amyloid fibrils to self-assemble and were based on a specific sequencing of prion proteins. These artificial amyloids demonstrate catalytic activities and have several advantages over natural enzymes, including modularity, flexibility, stability, and ease of use. Recently, researchers discovered that these molecules can effectively bind to metal ions and act as storage elements for metal and metalloenzymes.
“These peptides were particular, since they did not contain the typical amino acids, such as histidine, which is often considered essential for the coordination of metal ions in enzymes, and which were thought to be essential for catalytic activity. In contrast, they were enriched with residues from tyrosine, an element which although less known in this context, can also have the unique capacity of binding to metal ions if it finds itself in the correct structural context. Tyrosine’s ability to do so is what we used to create our nanozymes,” Salvador Ventura points out.
The results of the study have wide-ranging applications. Firstly, nanozymes exhibit excellent stability and can be employed for environmental remediation purposes, including wastewater treatment and decontamination of soils, due to their remarkable ability to sequester metal ions. Secondly, they can act as metalloenzymes, catalyzing reactions in conditions where current enzymes would be incapable of functioning due to their instability. This creates exciting new possibilities for biotechnology research, such as catalyzing reactions in extreme temperatures and pH values.
Researchers have developed a minimalistic variant of a carbonic anhydrase enzyme that can efficiently store CO2 emitted by greenhouse gases. They are convinced that their enzyme is highly capable and can be produced at a much lower cost than natural enzymes.
Researchers have developed new nanozymes by exploring the catalytic activity of short, low-complexity peptides that self-assemble into structures similar to amyloids. This hypothesis suggests that such structures acted as the primal ancestral enzymes, playing a vital role in the origin of life.
“Showing that these molecules have catalytic action without the need for conventional histidine-based coordination represents a significant change in how we understand the origin of catalytic activity at the start of life. We now know that this activity can be achieved if the ancestral peptides contain tyrosine. Therefore, we suggest that it is highly probable that the ancestral enzymes based on amyloids also used this second amino acid in their chemical reactions,” Salvador Ventura concludes.
The potential of minimal molecules in capturing CO2 is a promising development in the battle against climate change. By leveraging the unique properties of these molecules, we can create cost-effective solutions to reduce CO2 emissions and safeguard our planet. With further research and development, minimal molecules can become a potent tool in combating global warming and the consequences of climate change. With the appropriate resources and commitment, we can build a greener, cleaner, and more sustainable future for generations to come.
POPULAR
- NASA predicts the Sun's corona behavior, revealing its mysteries using advanced computational methods
- Australia on track for unprecedented, decades-long megadroughts: Supercomputer modeling raises concern
- Revolutionizing precision agriculture: The impact of Transformer Deep Learning on water, energy demands

 How to resolve AdBlock issue?
How to resolve AdBlock issue?